ABSTRACT
OBJECTIVE: This study evaluated the effects of low level laser (LLL) therapy in the healing of third degree burning wounds in diabetic and non-diabetic Wistar rats.
METHODS: The diabetes was experimentally induced with streptozotocine 14 days before the burning injury induction. The rats suffered the induction of third-degree burning injury and were divided into four groups as follows: control group; non-diabetic treated group; diabetic group; diabetic treated group. All animals received occlusive bandages during the experimental days. The treated animals received the following treatment in alternate days: diode GaAlAs laser (650 nm/ 12 mW), fluency of 3 J/cm2 until the 7th experimental day followed by 6 J/cm2 from the 7th day until the euthanasia day. The burning wounds were morphometrically, macroscopically and microscopically evaluated at 3, 7, 14, 21 and 30 days after the induction.
RESULTS: The wound contraction was significantly higher in all experimental days in treated groups when compared to the diabetic and non-diabetic control groups. Microscopically, there was a significant increase in angiogenesis and in fibrogenesis during the proliferative stage in the treated groups.
CONCLUSION: Therefore, we conclude that LLL therapy favored the tissue healing process with 3 J/cm2 dosage for the inflammatory stage and with 6 J/cm2 dosage for the proliferative and remodeling ones, accelerating the burning wound contraction and improving the healing process.
Keywords:
Burns. Diabetes Mellitus. Low-Level Light Therapy. Pathology.
RESUMO
OBJETIVO: Este estudo avaliou os efeitos da terapia a laser de baixa intensidade (LBI) na cicatrizaçao de feridas por queimadura de terceiro grau em ratos Wistar diabéticos e nao diabéticos.
MÉTODOS: A diabete foi induzida experimentalmente com estreptozotocina 14 dias antes da induçao da lesao por queimadura. Os ratos sofreram a induçao da lesao por queimadura de terceiro grau e foram divididos em quatro grupos: grupo controle; grupo tratado nao diabético; grupo diabético; grupo tratado diabético. Todos os animais receberam curativos oclusivos durante os dias experimentais. Os animais tratados receberam o seguinte tratamento em dias alternados: laser diodos GaAIAs (650 nm/12 mW), fluência de 3 J/cm2 até o sétimo dia experimental, seguido por 6 J/cm2 a partir do sétimo dia até ao dia eutanásia. As feridas por queimaduras foram avaliadas morfometricamente, macro e microscopicamente em 3, 7, 14, 21 e 30 dias após a induçao.
RESULTADOS: A contraçao da ferida foi significativamente maior em todos os dias experimentais nos grupos tratados quando comparados com os grupos controle diabéticos e nao diabéticos. Microscopicamente, houve aumento significativo na angiogênese e na fibrogênese durante a fase proliferativa nos grupos tratados.
CONCLUSOES: Concluímos que a terapia LBI favoreceu o processo de cicatrizaçao do tecido com dosagem de 3 J/cm2 na fase inflamatória e com dosagem de 6 J/cm2 nas fases proliferativa e de remodelaçao, acelerando a contraçao da ferida por queimadura e melhorando o processo de cicatrizaçao.
Palavras-chave:
Queimaduras. Diabetes Mellitus. Terapia com Luz de Baixa Intensidade. Patologia.
INTRODUCTION Tissue repair mechanisms may be prolonged when endogen and exogenous alterations occur concomitantly in the same patient such as diabetes mellitus (DM) and third degree burning wounds. These burning wounds are considered complex injuries that require a distinct treatment which involves a multidisciplinary team and varied approaches to obtain the healing success
1. LASER is an acronym for "Light Amplification by Stimulated Emission of Radiation" and is a device of electromagnetic radiation (EMR) with specific characteristics applied to each tissue and to each wave length in which the anti-inflammatory, analgesic, tissue repair, pigmentation and surgical effects are highlighted
2.
High complexity wounds such as burning wounds in diabetic patients are a public health issue as they affect all classes of the worldwide population. Burning wounds are a great challenge for health professionals and for the public health system around the world as it demands specialized human, material and financial resources
3. Third degree burn wounds are considered, by its extension, high complexity injuries which present slow wound closure. The laser therapy is a resource that involves the appliance of a monochromatic light of low energy speeds up the healing process avoiding its exposure to agents that may compromise the tissue repair
2.
The Ministry of Health of Brazil reports that the burning wounds are in fourth place of the ranking of death caused by injury and it is estimated that at least 1,000,000 people suffer some kind of burning wound per year in this country
4.
There are several different approaches to treat burning wounds when in the tissue repair stage such as polymers, phytoterapic extracts, mineral compounds, physical resources such as low level laser (LLL), therapeutic ultrasound (US), light emitting diode (LED) and hyperbaric chambers. Some of these approaches are widely used in clinic consultations and result in positive outcomes
1.
Some studies using experimental models identified the beneficial effects of LLL in the treatment of burning wounds in diabetic and non-diabetic rats. These studies show that the capacity of the laser light to penetrate within the injured tissue favors the contraction of the wound, angiogenesis, fibroblasts proliferation and, consequently, a greater collagen deposition in situ
5,6. However, there is great diversity in the parameters of LLL use, for instance, wave length, energetic dosages and applying form.
Therefore more detailed studies on the effect of factors that interfere in the wound healing process such as the concomitancy of diabetes mellitus and burning wounds should be performed. Thus the aim of this study was to evaluate the effect of low level laser of 650nm in the healing process of third degree burning wounds experimentally induced in diabetic and non-diabetic rats through macroscopic, microscopic and morphometric analysis and the quantification of collagen.
METHODS
Samples and ethic aspects An experimental prospective longitudinal study was performed with 100 Wistar rats approved by the Animal Use Ethics Committee/CEUA-PRPPG-UFG, protocol number 007/12.
Three animals were housed in each cage. They received water and autoclaved commercial ration ad libitum and the bed changes were performed twice a week. The animals were carefully handled, always by the same researcher and in the morning period supervised by a veterinarian physician. An adaptation of the animals in the proposed environment was performed so as to prioritize their well being.
Experimental groups Female rats with 60-75 days of age and weighting 200-250 g were used. The animals were divided into four groups of 25 animals each as follows: control group (CG); diabetic group (DG); nondiabetic treated group (NDTG) and diabetic treated group (DTG). Five animals from each group were euthanized at 3, 7, 14, 21 and 30 days after the induction of the third degree burning wound for the analysis of the macroscopic, microscopic and morphometric parameters of the wound.
The euthanasia of the animals was performed through a peritoneal lethal injection of anesthetics used for the lesion induction but in the concentration of 1 ml/g of Ketamine 10% and Xilazine 2%.
All analyses were performed by a researcher who did not know the groups distribution.
Protocol for Type I Diabetes Mellitus (DM) induction The rats received an intraperitoneal injection of streptozotocine dissolved in citrate buffer 0.1M, pH=4.5, 40 mg/kg of weight to induce DM
7. The DM was confirmed through the glucose dosage by the Biocheck device and all animals who received streptozotocine and presented glycemia higher than 200 mg/dl were considered diabetic. The weight and glycemia were dosed before, 24 and 72 hours after the DM induction and in the day of the euthanasia. The DM induction was performed 14 days before the third degree burning wound induction.
After the DM confirmation a histopathologic analysis of the central arteriole from the white pulp of the spleen was performed aiming the detection of vascular alterations induced by this disease. The spleen of the animals (n=3) was removed at 2, 15 and 30 days after the DM induction and the tissues were processed for paraffin inclusion and two slides of 4 micrometer width each were stained with hematoxilin & eosin (HE) and picro-sirius. The alterations of the arteriole width were quantified through analysis with the Image J software (NIH) version 1.3.1.
Lesion protocol At day 0 the animals were anesthetized with 0.01ml/g of Ketamine 10% and Xilazine 2% via intraperitoneal. Afterwards a depilation of the dorsal region of the animal was performed and the third degree thermic injury induced by the immersion of this region in water at 95ºC for 14 seconds
8.
During the treatment period the animals received daily occlusive and sterilized bandages. Initially a cleaning of the wound with physiologic solution was performed followed by a topic application of silver sulfadiazine ointment. All animals received an occlusive bandage. A veterinarian physician supervised the injured animals during the animal hygiene, aggressiveness, posture and answer to handling were observed.
At the second day after the injury induction the surgical debridement of the wounds was performed
8.
Laser application The laser device used in this study was developed by Carci Indústria e Comércio de Aparelhos Cirúrgicos e Ortopédicos Ltda (Americanópolis - Sao Paulo - SP - Brasil), Model LASERMED 4098 with an automatic emission pen of continuous visible red of 650 nm and 12 mWatts, GaAlAs MOCVD (Energy=0.3 J; spot area=0.1 cm
2; emission potency=12 mW). For the LLL (low level laser) therapy treatment the burned areas were divided into four equal quadrants and each one of the quadrants was in contact with the light source emitted by the laser with 3 J/cm² dosage until seven days and 6 J/cm² during the remaining days. The time of irradiation was calculated by the ratio between the energy and emission potency. The animals were gently manipulated and stabilized by the researchers in a surgical bed in ventral decubitus with the members in extension and the laser application was performed in a punctual mode and perpendicularly to the injury bed, in alternated days. Each field received the light emission in a uniform punctual mode and with the same established parameters.
Macroscopic evaluation and wound contraction analysis In the established experimental days the stages of the inflammatory process were evaluated, i.e., inflammation, proliferation and maturation. The following parameters were analyzed: necrosis, crust, granulation tissue and contraction.
The wound contraction analysis was performed through the photography of the injuries through a digital camera attached to a tripod and at a constant distant of 20 cm from the lesion. Afterwards the images were analyzed through the Image J software (NIH) version 1.3.1.
The area of the wound was surrounded using the Image J software (NIH) by a researcher who did not know which treatment was being analyzed. Afterwards the contraction (CD) was calculated by the following equation: CD=[(area D
0 - area D')x100]/ area D°, where D° represents the day of injury induction and D' the corresponding experimental day (3, 7, 14, 21 or 30).
Microscopic evaluation The injury tissue was removed through biopsy procedure, processed to paraffin inclusion, sliced into 4 micrometers width and stained by hematoxilin & eosin (HE). After the animal euthanasia a full thickness skin (0.3 cm) was excised from the injured area with a scalpel. The removed skin sample contained the epidermis, dermis, subcutaneous fat, panniculus carnosus and the muscle layer. The samples from cutaneous lesions were collected as to include the skin adjacent to the wound edges and all the scar tissue in its depth.
The general pathologic processes were analyzed in the dermis of the injury. This was a semi-quantitative analysis as follows: absent (A) (score=0); discrete (D) with 1 to 25% of the fragment (score=1); moderate (M) from 26 to 50% of the fragment (score=2); and accentuated (Ac) above 51% of the fragment (score=3).
The pathologic processes analyzed were necrosis, fibrin, polimorphonuclear (PMN) cells infiltration, mononuclear (MN) cells infiltration, angiogenesis and fibroblasts quantification.
The collagen quantification was performed from skin fragments fixed and blocked in paraffin, stained by picro-sirius and counter stained by hematoxilin at 14, 21 and 30 days of experiment. The slides were analyzed through a polarized light microscopy with the 10x objective, photographed and the digital images were directed to the morphometric analysis which was performed in 20 fields through the Image J software, corresponding to the whole area of the slide. The images of the collagen areas were identified and converted into pixels by the software which resulted in a percentage of collagen from analyzed slide.
Statistical analysis All variables were tested regarding its normal distribution and homogenous variance through the Sigma Stat 3.2 software. When the distribution was considered normal and with homogenous variance the student t-test was used. When the distribution was not normal the Mann Whitney test was used. The differences were considered significant when
p<0.05. The number of animals used in this study was determined as to respect the animal experimentation principles and as to use the minimal amount of animals that allow an adequate statistical analysis
9.
RESULTS
Analysis of DM induction Aiming the evaluation of the compromise of the microvascularization in DM a pilot study was performed with three Wistar rats who suffered an experimental induction of DM. The animals submitted to the diabetic induction and who presented glycemic levels higher than 200 mg/dl in the evaluation period (24 and 72 hours, 7 and 15 days) were euthanized and had their spleen removed. In this organ transversal slices were made for the microscopic evaluation of the central arteriole from the white pup. In this evaluation was possible to observe a progressive increase in thickness of the wall of the central arteriole. Also this material showed negative results in PAS and picro-sirius staining which demonstrates that the observed increase is due to hyperplasia.
Macroscopic evaluation and wound contraction analysis The animals treated with laser presented a significant increase in the wound contraction (
p<0.05) when compared to the non-treated groups, both the non-diabetic as the diabetic groups (Table 1).
Microscopic analysis There was a significant increase (
p<0.05) in angiogenesis in both diabetic and non-diabetic treated groups when compared to the control groups at the third experimental day. At the fourteenth experimental day was also possible to observe a significant difference (
p<0.05) in angiogenesis and in fibroblasts quantification in the diabetic treated group in comparison to the diabetic control one. At the twenty first experimental days the statistical difference was observed on the fibroblasts quantification in the diabetic treated group in comparison to the diabetic control group. At all other experimental days the pathologic processes analyzed did not present statistical significance between groups (Figure 1) (Table 2).
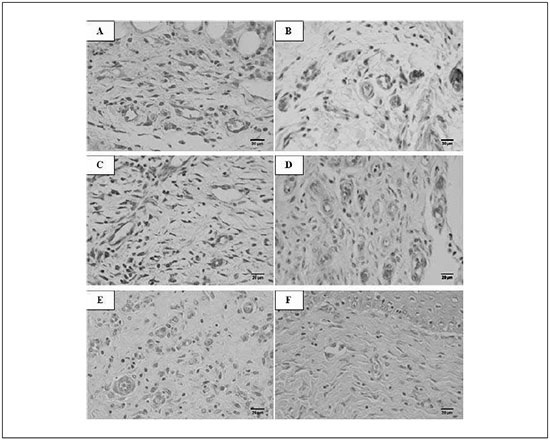
Figure 1 - Photomicroscopy of third degree burning wound in diabetic rats. A, C and E diabetic control group at 3, 14 and 21 experimental days, respectively. B, D and F diabetic treated group at 3, 14 and 21 experimental days, respectively. In B there is greater angiogenesis than in A. In D there is greater angiogenesis and fibroblasts quantification than in C. And in F there is greater fibroblasts quantification than in E. (HE, scale bar=20µm)
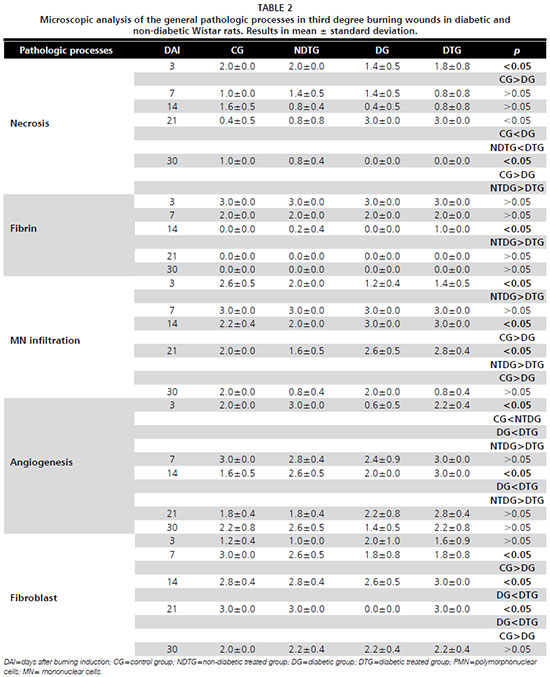
The collagen deposit was significantly higher in the laser treated groups (diabetic and non-diabetic) when compared to the control groups (diabetic and non-diabetic) (Table 3). There was no statistical difference in the polimorfonuclear infiltration comparison between the groups (data not shown).
The non-diabetic control presented more accentuated necrosis (
p<0.05) than the diabetic control group at 3 days post injury induction while the LLL treated groups did not present a statistical difference in necrosis intensity. At 21 and 30 days post injury induction when comparing the control groups and the treated groups was possible to observe a significant difference (
p<0.05) in the necrosis intensity which was increased in the diabetic groups at 21 days and in the non-diabetic groups at 30 days.
The diabetic treated group presented significantly higher intensity of fibrin (
p<0.05) when compared to the non-diabetic treated group at 14 days post injury induction.
The mononuclear infiltration was significantly more accentuated in the non-diabetic treated group (
p<0.05) when compared to the diabetic treated group at 3 days post injury induction. However, at 14 days post injury induction the statistical difference (
p<0.05) was observed when comparing the control groups in which the diabetic one presented more infiltration than the non-diabetic one. Interestingly this difference was not observed in the LLL treated groups at the same experimental day. At 21 days post injury induction the statistical difference was observed when comparing the control groups and the treated ones (
p<0.05).
The angiogenesis was increased in the non-diabetic treated group when compared to the diabetic treated one at 3 days post injury induction, however at 14 days it was increased in the diabetic treated one (
p<0.05).
The fibroblast intensity was increased in the non-diabetic control group when compared to the diabetic control one (
p<0.05) at 7 and 21 days post injury induction. Such statistical difference was not observed in the LLL treated groups at the same experimental day.
DISCUSSION This study brings an innovative approach for the treatment of burning wounds in diabetic experimental model with rats which is the use of the occlusive bandage during the whole period of treatment associated to the surgical debridement of the wound and the variation in the dosimetry accordingly to the inflammatory phase of the healing process. The surgical debridement performed in this study evidently contributed to the reduction of necrosis in the wounds.
The great majority of studies that used LLL therapy did not adopt this procedure leading to an accentuated necrosis of the wound even in the late phase of the healing process
10. The necrosis/crust removal allowed that the light could be emitted in a more direct form and achieved greater depths in the wounded site. The occlusive bandage was adopted as to maintain the hydration of the wound which minimized the crust formation, traumas and contamination of the wound bed due to the contact with other animals in the cage.
This study also evaluated the morphometric, macroscopic and microscopic alterations in third degree burning wounds healing process in diabetic and non-diabetic Wistar rats treated with LLL therapy. The wound contraction rate was significantly higher in LLL treated groups in comparison to the control groups. The wound contraction rates reported by other authors that also used one wavelength of the LLL therapy are similar to the ones found in this study
6,10,11. These results indicate that even in the presence of factors that interfere in the healing process such as diabetes mellitus the LLL therapy is effective.
In the inflammatory or exudative stage, in the present study, some of the analyzed pathological processes present a significant difference between the groups. Other authors demonstrated that LLL therapy has positive effect at the early phases of wound healing process
12 in accordance to the findings of our study. However, there was no report on the literature about the changes in dosimetry during the treatment and the effect of the occlusive bandage on the wound healing process when comparing diabetic and non-diabetic experimental groups.
In the analysis of the proliferative stage was possible to observe a significant difference both in angiogenesis and in the presence of fibroblasts, at 14 days after burning induction in diabetic animals who received the LLL therapy when compared to the diabetic control group. According to the literature angiogenesis occurs simultaneously to the fibroplasia in which the new blood vessels will give support to the new interstitial matrix that is being formed
13.
The LLL therapy promotes the inflammatory modulation in the irradiated site and the presence of depurative cells stimulates the release of substances such as fibroblasts growth factor (FGF) and vascular endothelial growth factor (VEGF). Therefore the formation of new blood vessels supplies this new environment with more oxygen and nutrients favoring the migration of fibroblastic cells which contributes to the healing process
14.
Angiogenesis and moderate to accentuated fibroblasts quantification was also observed in animals treated with one wavelength of LLL therapy
15. The increase in angiogenesis and fibroblasts quantification may be related to the fact that low level lasers stimulate the oxidative mechanism of mitochondria especially when submitted to 665nm wavelength
16.
Both angiogenesis and fibroblasts migration in the burning wound site favored the significant increase in collagen deposit in the region especially in the LLL therapy treated groups. Similarly, other authors describe that the photomodulation of the laser therapy is essential for the fibroblastic migration and consequently to the increase in collagen deposit
6,7,11,15. It has been reported that the LLL therapy lead to better results in the healing process of diabetic animals comparing to non-diabetic ones
10. Other authors have reported that the collagen concentration on the wound bed was also greater in the diabetic animals treated with LLL therapy when compared to the non-diabetic ones
17. These data are very similar to the ones found in this study, especially at 14 days after the burning induction.
Also, it has been reported that LLL therapy in wound repair in diabetic rats leads to increase in fibroblasts and collagen when compared to the diabetic controls
16. Furthermore LLL therapy induces the conversion of the diabetic wound healing into normal healing such as demonstrated in our studies
10.
In this study was possible to observe a progressive thickness of the wall of the central arteriole of the white pulp of the spleen in diabetic animals. Also it has been confirmed the arteriole thickness in histological material of patients with diabetic foot and that the treatment of such wounds should be performed with therapeutic methodologies that stimulate the biological components of the healing process
18.
CONCLUSION Therefore we conclude that based on the evaluation of the results in this study accordingly to the used methodology the LLL-650nm therapy stimulates the angiogenesis in the inflammatory stage and fibrogenesis in the proliferative stage contributing to the remodeling stage and repair of the injured tissue. Furthermore there was little effect of the LLL therapy on non-diabetic animals and promising effects on diabetic ones. Therefore a clinical application o LLL therapy in diabetic individuals with burn injuries is recommended in the early phases of the healing process.
ACKNOWLEDGMENTS Finantial support: Fundaçao de Amparo à Pesquisa do Estado de Goiás (FAPEG). The authors would like to thank CARCI - Indústria e Comércio de Aparelhos Cirúrgicos e Ortopédicos Ltda for believing in this study and for providing the equipment used in it; Hospital de Queimaduras de Goiânia, especially to Dr. Dr Nelson Piccolo for the incentive.
REFERENCES 1. Coltro PS, Ferreira MC, Batista BP, Nakamoto HA, Milcheski DA, Tuma Júnior P. Role of plastic surgery on the treatment complex wounds. Rev Col Bras Cir. 2011;38(6):381-6.
2. Brassolatti P, Bossini PS, Oliveira MCD, Kido HW, Tim CR, Almeida-Lopes L, et al. Comparative effects of two different doses of low-level laser therapy on wound healing third-degree burns in rats. Microsc Res Tech. 2016;79(4):313-20.
3. Shoham DA, Mundt MP, Gamelli RL, McGaghie WC. The Social Network of a Burn Unit Team. J Burn Care Res., 2015;36(5):551-7.
4. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Queimadura. In: Guia de vigilância epidemiológica. Brasília: Ministério da Saúde; 2014.
5. Bayat M, Abdi S, Javadieh F, Mohsenifar Z, Rashid MR. The effects of low-level laser therapy on bone in diabetic and nondiabetic rats. Photomed Laser Surg. 2009;27(5):703-8.
6. Fiório FB, Albertini R, Leal-Junior EC, de Carvalho Pde T. Effect of low-level laser therapy on types I and III collagen and inflammatory cells in rats with induced third-degree burns. Lasers Med Sci. 2014;29(1):313-9.
7. de Almeida SM, Ferreira RI, Bóscolo FN. Influence of irradiation on collagen content during wound healing in diabetic rats. Pesq Odontol Bras. 2002;16(4):293-8.
8. de Moraes JM, Eterno de Oliveira Mendonça D, Moura VB, Oliveira MA, Afonso CL, Vinaud MC, et al. Anti-inflammatory effect of low-intensity laser on the healing of thirddegree burn wounds in rats. Lasers Med Sci. 2013;28(4):1169-76.
9. Damy SB, Camargo RS, Chammas R, Figueiredo LF. Fundamental aspects on animal research as applied to experimental surgery. Rev Assoc Med Bras. 2010;56(1):103-11.
10. Al-Watban FA. Laser therapy converts diabetic wound healing to normal healing. Photomed Laser Surg. 2009;27(1):127-35.
11. Fekrazad R, Nikkerdar A, Joharchi K, Kalhori KAM, Abbas FM. Effect of laser photostimulation on the healing of third-degree burn wounds in rats. J Arch Mil Med. 2014;2(3):1-6.
12. Ma H, Li YX, Chen HL, Kang ML, Liu TCY. Effects of low-intensity laser irradiation on wound healing in diabetic rats. Int J Photoenergy, 2012;(2012):1-7.
13. Balbino CA, Pereira LM, Curi R. Mecanismos envolvidos na cicatrizaçao: uma revisao. Rev Bras Ciênc Farm. 2005;41(1):27-51.
14. Rocha JCT. Terapia laser, cicatrizaçao tecidual e angiogênese. Rev Bras Promoç Saúde. 2004;17(1):44-8.
15. Gupta A, Keshri GK, Yadav A, Gola S, Chauhan S, Salhan AK, et al. Superpulsed (Ga-As, 904 nm) low-level laser therapy (LLLT) attenuates inflammatory response and enhances healing of burn wounds. J Biophotonics. 2015;8(6):489-501.
16. Dancáková L, Vasilenko T, Kováč I, Jakubčová K, Holly M, Revajová V, et al. Low-level laser therapy with 810 nm wavelength improves skin wound healing in rats with streptozotocin-induced diabetes. Photomed Laser Surg. 2014;32(4):198-204.
17. Oliveira PC, Pinheiro AL, Reis Junior JA, de Castro IC, Gurgel C, Noia MP, et al. Polarized light (λ400-2000 nm) on third-degree burns in diabetic rats: immunohistochemical study. Photomed Laser Surg. 2010;28(5):613-9.
18. Meireles GC, Santos JN, Chagas PO, Moura AP, Pinheiro AL. Effectiveness of laser photobiomodulation at 660 or 780 nanometers on the repair of third-degree burns in diabetic rats. Photomed Laser Surg. 2008;26(1):47-54.
Part of this work is the result of the Doctoral Thesis entitled: "Efeito do tratamento com laser de baixa potência e ultrassom na cicatrizaçao de feridas em ratos com e sem diabetes" authored by Marcelo Silva Fantinati, defended at the Post-Graduate Program in Tropical Medicine and Public Health of the Tropical Institute of Pathology and Public Health of the Federal University of Goiás.
Recebido em
2 de Maio de 2016.
Aceito em
11 de Julho de 2016.
Local de realização do trabalho: Tropical Institute of Pathology and Public Health of the Federal University of Goiás, Goiânia, GO, Brazil.
The authors declare that they have no financial conflict of interest.